Status: Graduate Student My research interests over the past four years can be broken up into the following categories (hover over images for description):
The steep scaling of computational cost with molecular size limits the application of the most accurate quantum chemical methods to systems with relatively few atoms.
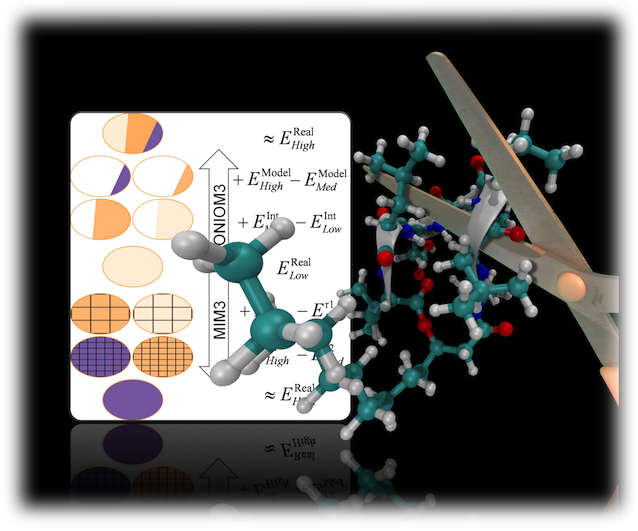
By providing a methodological framework to couple accurate electronic structure methods with more computationally tractable methods, hybrid electronic structure methods (such as ONIOM) have been extremely powerful tools for obtaining accurate computational results for large to very large molecular systems. The ability of a hybrid electronic structure method to provide accurate and chemically meaningful results is often determined by the means in which the high-level and low-level wavefunctions interact. In the ONIOM method, the energy is obtained by the following expression: Where ERL, EMH, and EML are the real:low energy (energy of the full molecule at the low-level of theory), the model:high energy (energy of the "active site" at the high-level of theory), and the model:low energy (energy of the "active site" at the low-level of theory), respectively.
Thus the ONIOM energy can be thought of as a high-level correction to an "active site" of a large molecule. As all three separate calculations are performed on a well-defined molecule, the model system ("active site") calculations are typically conducted without any information about the rest of the molecule. We have developed energy and gradient methods for embedding the model system calculations into a field of point-charges obtained from the real system sub-calculation. This provides an explicit coupling between the real system and the model systems. The zeolite image is shown as an example of the types of systems currently being investigated using the newly developed ONIOM methodologies.
As with the ONIOM methodology, fragmentation methods aim to address the steep computational scaling of our most useful theoretical methods. Unlike ONIOM, however, fragmentation methods approximate the energy of the full molecular system with the target level of theory. We have recently developed a new extrapolated fragment-based approach, termed molecules-in-molecules (MIM), for accurate energy calculations on large molecules. In this method, we use a multilevel partitioning approach coupled with electronic structure studies at multiple levels of theory to provide a hierarchical strategy for systematically improving the computed results. In particular, we use a generalized hybrid energy expression, similar in spirit to that in the popular ONIOM methodology, that can be combined easily with any fragmentation procedure. The basic idea is shown here:
In the current work, we explore a MIM scheme which first partitions a molecule into non-overlapping fragments and then recombines the interacting fragments to form overlapping subsystems. By including all interactions with a cheaper level of theory, the MIM approach is shown to significantly reduce the errors arising from a single level fragmentation procedure.
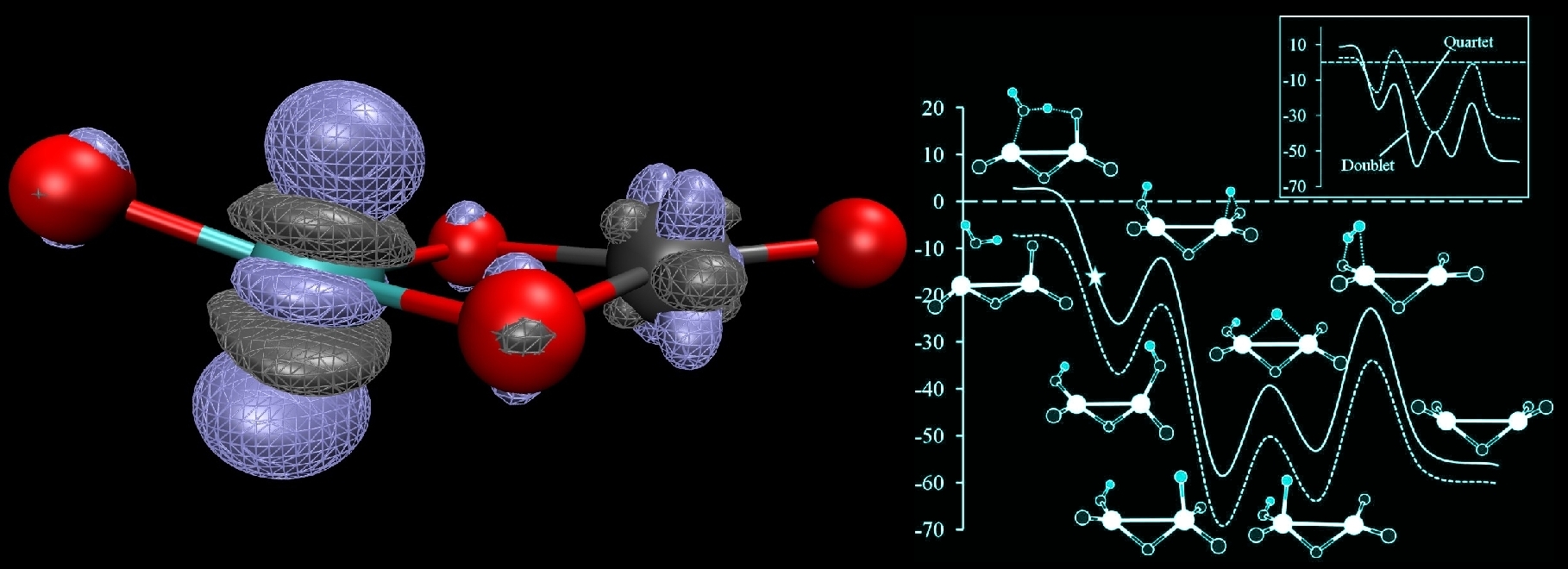
Using both newly developed and currently available computational methods, we study both structural properties and reactions of transition metal oxides and sulfides. In a collaborative effort with Professor Caroline Jarrold, we probe the reactive interactions between small molecules such as methane and water with transition metal oxide clusters. On the chemical front, we have obtained mechanistic explanations of several interesting experimental observations, ranging from novel activation pathways of methane using molybdenum oxide clusters, to the production of H2 and sequential oxidation of tungsten oxide clusters upon reaction with water.
On the more physical front, we have used computations to resolve ambiguities in experimental photoelectron spectroscopy of metal oxide systems. We are currently working to further elucidate the chemical and physical importance of various aspects of these cluster systems such as the role of the metal's identity and the effect of replacing oxygen with other atoms such as sulfur. Working with Larry Curtiss at Argonne National Laboratory, we are developing new composite model chemistries which are capable of computing accurate thermochemical properties of transition metal complexes. The performance of these methods depends on the ability to separate extensions in the description of electron correlation and the one-electron basis set size. Therefore, an understanding of the correlation energy convergence patterns for transition metal complexes is essential.
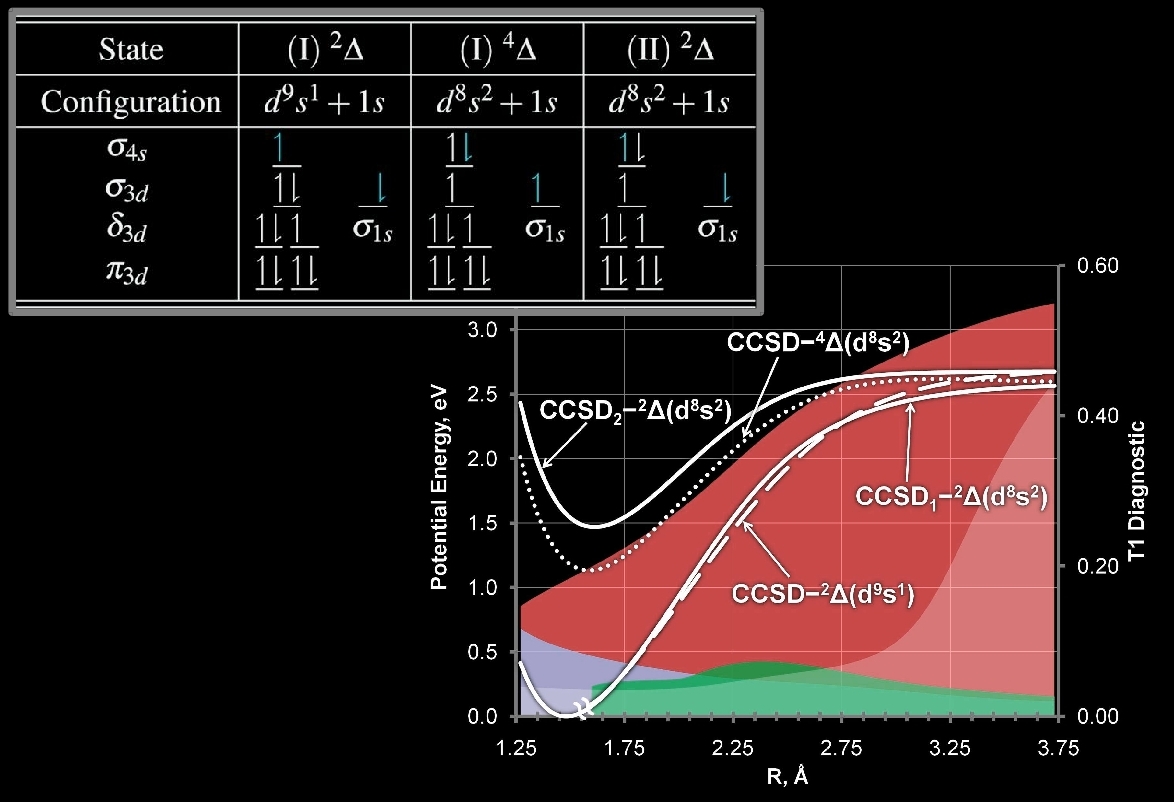
In this direction, we have recently explored a "simple" diatomic molecule, NiH, in a case-study fasion to elucidate the interesting details of its behavior when different electronic structure methods are used. In this work, we observed multiple solutions to the CCSD equations with the lowest energy CCSD solution describing a completely different electronic state than the underlying HF reference wavefunction. This renders a black-box treatment of the molecule particularly difficult. However, use of the BD method avoids these problems as the underlying reference is also optimized. The potential energy curves for the multiple CCSD curves are shown below:
Major(s): Physical Chemistry
Minor(s): Inorganic Chemistry
Current Position: Assistant Professor at Virginia Tech
● QM:QM Development
EONIOM = ERL + (EMH ‒ EML).
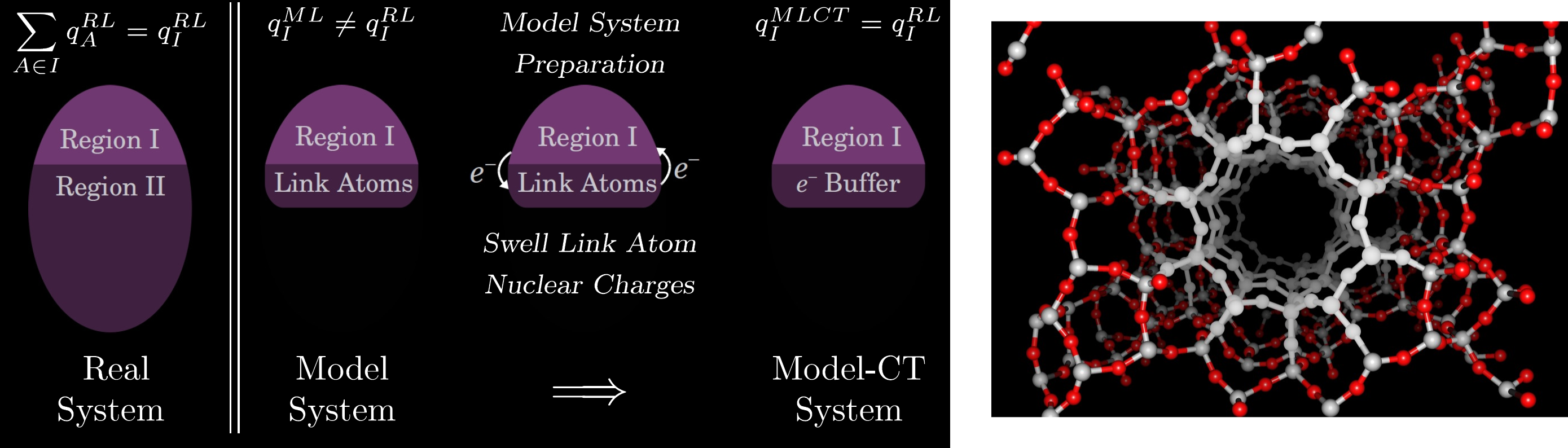
More recently, we have also developed a general scheme for including the effects of charge transfer across the ONIOM boundaries at the high-level of theory. As the model system is required to have an integer number of electrons, the model:high and model:low sub-calculations may have a significantly different number of electrons than what is present in the real:low sub-calculation. By treating the link-atoms as an electron buffer region, we iteratively optimize the link-atom nuclear charges to reproduce the charge distribution of the full, real-system calculation. This is illustrated in the scheme below:
● Fragmentation Methods
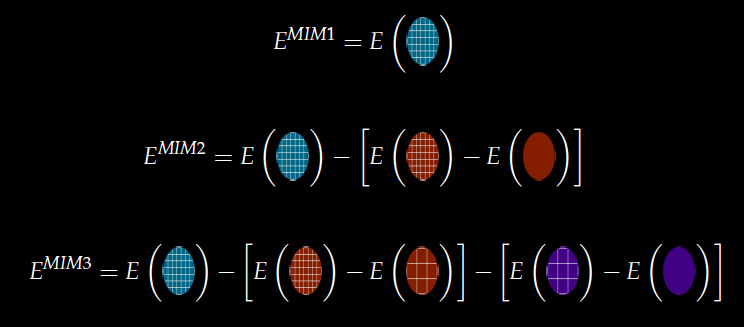
● Gas-Phase Cluster Chemistry
● Transition Metal Thermochemistry